Abstract
Introduction: Patients with the Philadelphia negative myeloproliferative neoplasms (MPN) have an increased risk of thromboembolic events, ischemic cerebrovascular events in particular. The somatic mutation JAK2V617F is associated with MPN with a prevalence of 96% in polycythemia vera (PV), 55% in essential thrombocythemia (ET), and 65% in primary myelofibrosis (PMF). The JAK2V617F mutation is found to be an independent risk factor for thrombosis in patients with MPN. However, whether the JAK2V617F mutation is a risk factor for arterial thrombosis in the absence of an MPN diagnosis is still uncertain.
The sensitivity of the assay used for detection is crucial when assessing the presence of the JAK2V617F mutation. A higher sensitivity enables detection of lower allele burdens. This is illustrated by the varying prevalences (0.1-3.1%) in background population studies. In the Danish population study GESUS of approximately 20.000 individuals, the JAK2V617F mutation was detected by droplet digital PCR (ddPCR) with an assay sensitivity of 0.009%. The GESUS study found a JAK2V617F prevalence of 3.1%.
Similarly, prevalence studies of JAK2V617F mutation in stroke have varying detection limits from 0.01-5% mutant alleles. The vast majority of these studies are retrospective studies in younger subpopulations of stroke patients.
We aimed to investigate the prevalence of the JAK2V617F mutation in a prospective design of consecutively enrolled unselected patients with ischemic stroke or transient ischemic attack by the highly sensitive ddPCR.
Method: We recruited 90 consecutive patients with ischemic stroke or transient ischemic attack during admission at the Department of Neurology, Zealand University Hospital, Denmark. A blood sample was taken within 7 days of the onset of symptoms and screened for the JAK2V617F mutation by ddPCR. Patients unable to give informed consent were excluded from the study.
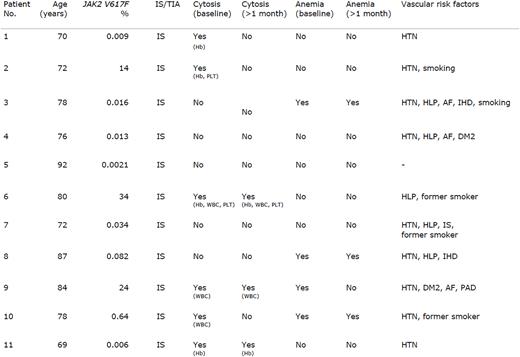
Results: Out of 90 patients (mean age 71, range 46-92; female 37%) with ischemic stroke or transient ischemic attack, the JAK2V617F mutation was found in 11 patients (12.2%). Allele burdens ranged from 0.0021 to 24. All 11 patients suffered an ischemic stroke. Six out of 11 patients with the JAK2V617F mutation had elevated blood cell levels i.e. erythrocytosis, thrombocytosis, and/or leukocytosis at the time of recruitment. Only three patients had continuously elevated blood cell levels after 1 month. Patient no. 6 with a JAK2V617F mutation allele burden of 34% had blood cell levels meeting the diagnostic criteria for MPN and was diagnosed with ET. Patient no. 2 had a JAK2V617F allele burden of 14% and increased levels of hemoglobin and platelet count, which both normalized spontaneously after 1 month. The patient was referred for a hematological workup and was found to have mild splenomegaly and a bone marrow biopsy showing myeloproliferative features. In light of the normal blood cell parameters, patient no. 2 was diagnosed with MPN-U. The patients with a JAK2V617F mutation are presented in table 1. Additional data will be presented at ASH.
Conclusion and implications: The present study reveals a JAK2V617F mutation prevalence (12.2%) in a stroke population that is substantially higher compared to the background population (GESUS) in the same region of Denmark, using the same assay. The GESUS study showed a JAK2V617F mutationprevalence of 3.1% and for individuals above age 60 a prevalence of 4.1%. Given the fact that the JAK2V617F mutation is present in patients with and without cytosis, our results highly suggest that the JAK2V617F mutation per se is a thrombogenic factor in ischemic stroke. This calls for an exploration of the pathophysiological mechanisms and potential treatments to prevent future thrombotic events in individuals with and without cytosis.
Table legend: Clinical and paraclinical characteristics of ischemic stroke patients with the JAK2V617F mutation. IS, ischemic stroke; TIA, transient ischemic attack; HTN, hypertension; HLP, hyperlipidemia; AF, atrial fibrillation; IHD, ischemic heart disease; DM2, diabetes mellitus type 2; Hb, hemoglobin; PLT, platelet count; WBC, white blood cells.
Disclosures
Hasselbalch:AOP Orphan Pharmaceuticals: Consultancy, Other: Data monitoring board; Novartis: Consultancy, Other: Grants.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal